Researchers have synthesized five new isotopes that could help knock stars down to Earth, bringing scientists one step closer to understanding how collisions between ultra-dense, dead stars can create heavy elements like gold and silver.
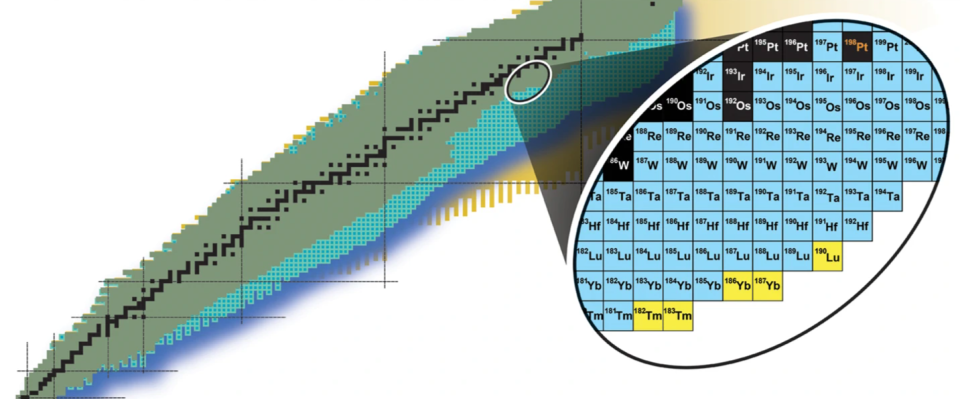
The isotopes are Thulium-182, Thulium-183, Ytterbium-186, Ytterbium-187, and Lutetium-190; This is the first time they were synthesized on Earth. Their creation took place at the Rare Isotope Beams Facility (FRIB) at Michigan State University (MSU) and represents a step towards the creation of atoms formed in the ultra-turbulent environment around the merger of dead stars on Earth, commonly known simply as neutron stars.
“This is the exciting part,” Alexandra Gade, FRIB scientific director and professor in MSU’s Department of Physics and Astronomy, said in a statement. “We are confident that we can get even closer to the nuclei that are important for astrophysics.”
Relating to: What happens when neutron stars collide? Astronomers may finally know
What is an isotope?
Each chemical element of the periodic table is defined by the number of protons in its atomic nucleus. For example, hydrogen always has one proton, helium always has two protons, and iron has 26 protons. Hydrogen cannot have two protons, iron cannot have 25 protons; If they were, they would no longer be hydrogen or iron.
But protons are joined by neutrons in the atomic nucleus, and the number of these particles can vary without changing the nature of an element. Nuclei containing varying numbers of neutrons are called isotopes of an element. So, iron isotopes include iron-54 with 26 protons and 28 neutrons, iron-56 with 26 protons and 30 neutrons, and iron-57 with 26 protons and 31 neutrons.
The five newly synthesized isotopes are exciting because they are not commonly found on our planet. In fact, they never even existed. to create before on our planet.
“This is likely the first time these isotopes have existed on the Earth’s surface,” Bradley Sherrill, MSU Distinguished Professor in the School of Natural Sciences and head of the FRIB Advanced Rare Isotope Separator Division, said in a statement. said. “I like to use the analogy of going on a road trip. We’ve been looking forward to going somewhere we’ve never been before, and this is the first step. We leave home and start exploring.”
Superheavy isotopes and superheavy elements
In general, stars can be thought of as nuclear furnaces that create the elements of the universe, starting with the fusion of hydrogen into helium and then fusing together to form nitrogen, oxygen, and carbon.
The most massive stars in our universe can process elements from the periodic table down to iron, but scientists believe that even these powerful stellar furnaces are not enough to create elements heavier than this. So what if two stars join their bakery? And in a pretty violent way?
The thing is that when massive stars die, they are left with iron cores that can no longer break down into heavier elements; The energy that supports these stars against their own gravitational effects pushing them inward also ends. This causes the cores to collapse, with the outer layers blasted away by powerful supernova explosions.
But this collapse can be stopped when the electrons and protons in these nuclei are converted into a sea of neutrons, which are prevented from being squeezed together by an aspect of quantum physics called “degeneracy”. If a stellar core has sufficient mass, this degeneracy pressure can be overcome, causing it to completely collapse and form a black hole. But sometimes there is not enough mass. These remain dead, extremely dense neutron stars.
Moreover, the end of this process does not mean the end of nuclear fusion if neutron stars exist in a binary system with another massive star and these stars eventually collapse to give birth to a neutron star. These ultra-dense stars, with masses between one and two times that of the Sun, emit ripples in space-time called gravitational waves as they squeeze around each other to a width of about 12 miles (20 kilometers).
These gravitational waves remove angular momentum from the system, causing neutron stars to come together and emit more gravitational waves at greater intensities. This continues until the two finally come together.
Given their extreme nature, it is not surprising that collisions of binary neutron stars create a very violent environment. This event, for example, spews out neutron-rich material, which is believed to be important for the synthesis of gold and other heavy elements.
This is because these free neutrons can be captured by other atomic nuclei in the environment through a rapid capture process, or what is called the “r-process”. These greedy atomic nuclei then become heavier, creating unstable superheavy isotopes. These unstable isotopes are expected to eventually evolve into stable elements that are lighter than superheavy elements like gold but still heavier than iron.
“It’s not certain, but people think all the gold on Earth is formed from neutron star collisions,” Sherrill said. In fact, the James Webb Space Telescope recently found the best evidence for the theory.
So how do we find out whether this process is definitely happening?
If scientists could recreate the superheavy elements involved in the r-process, they could better understand the formation of gold and other heavy elements. Unfortunately, the creation of thulium-182, thulium-183, ytterbium-186, ytterbium-187 and lutetium-190. These isotopes, created by firing a beam of platinum ions at a carbon target in the FRIB, may not be present in the debris of neutron star collisions, but their presence on Earth is certainly a step towards the creation on our planet of transient superheavy elements that lived for short periods of time. Look at the planet to see if it results in elements like gold.
RELATED STORIES:
— ‘Impossible’ neutron stars could explain strange flares
— How neutron star collisions filled Earth with gold and other precious metals
— Most powerful gamma-ray burst ever seen could help reveal how black holes are born
Ultimately, a better understanding of these newly created isotopes could also have important implications for nuclear physics.
“It’s not a big surprise that these isotopes exist, but now that we have them, we have colleagues who will be very interested in what we can measure next,” Gade concluded. “I’m starting to think about what we can do next in terms of measuring their half-lives, their masses, and other properties.”
The team’s research was published Thursday, February 15, in the journal Physical Review Letters.